Autocatalytic Hydroxylation of the Cα Atom in Proteins: Mechanism and Biological Role
The modified Proline
Post-translational modifications cause a significant increase in the diversity and functional complexity of proteins. In eukaryotic organisms, the posttranslational hydroxylation of proline (Pro) side chains by prolyl hydroxylases produces various forms of hydroxyproline (Hyp), a component of collagen, plant cell wall architecture, and key player in signaling processes linked to hypoxia response and physiological pathways associated with diseases such as cancer. In bacteria, Pro hydroxylation is understudied and occurs mainly on free Pro. One putative bacterial peptidyl-prolyl hydroxylase has been reported however for Bacillus anthracis although its substrate and biological role are presently unknown. The establishment of Pro hydroxylation as a crucial posttranslational modification (PTM) makes the elucidation of its full extent and its various biological roles an important issue, in particular since several recent studies imply that hydroxylation may be a significantly more extensive PTM than previously perceived.
An exotic post-translational modification
The scientific team of the OH-Cα project has recently discovered in polysaccharide deacetylases (PDAs) molecules (Fadouloglou et al., JACS 2017) an autocatalytic hydroxylation process of the Cα atom of Pro, in form of a Pro → 2-Hyp conversion.
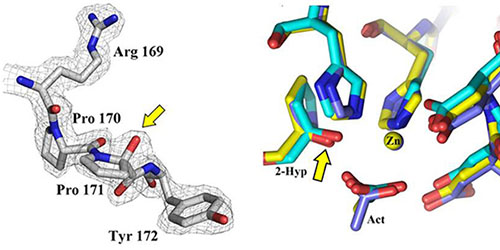
Fig.1: 2-Hyp sites in PDAs. Left: Detail from the crystal structure of the Bc1960 deacetylase (B. cereus) showing the 2-Hyp modification of residue Pro171. Right: Superposition of the active sites of three PDAs (Bc1960, Bc0361 and Bc0330). The Cα hydroxylation sites are indicated by the arrow, showing extensive structural homologies.
This autocatalytic process coexists in PDA active sites with the well-known deacetylation activity. These active sites exhibit extensive structural and sequence similarities across various enzymes (Fig. 1). The 2-Hyp residues are similarly positioned in the interior of the active site, with their hydrogen bonding networks being broadly conserved. Structural homologies and multiple sequence alignments reveal a relation between the conserved PDA sequence motifs (Fadouloglou et al., JACS 2017) and the site of the new PTM: The hydroxylation activity modifies with utmost specificity the highly conserved Pro of motif MT3 for which no role could be determined in the past.
Understanding the autocatalytic Cα hydroxylation of Pro: the OH-Cα project
The OH-Cα project has the following five general objectives:
- Α) To contribute to a detailed and comprehensive understanding of the mechanism of autocatalytic Cα hydroxylation of Pro residues by determining what constitutes a favorable environment for the Pro hydroxylation reaction in terms of the physicochemical properties of the closest active site residues.
- Β) To restore, through the introduction of mutations in the environment of the conserved Pro of the pseudoenzyme Ba3943 (Fig. 3), the network of interactions that control Cα hydroxylation in PDAs, so as to evaluate whether the basic structural requirements for the auto-catalytic Cα hydroxylation (some of them analyzed in the framework of the proposed project) have been sufficiently understood.
- C) To investigate and characterize the hypothetical mechanism of the Cα hydroxylation of glycine (Gly) residues.
- D) To screen protein structure databases such as the Protein Data Bank (PDB) archive, for the existence of autocatalytic Cα hydroxylation sites in protein families other than PDAs. Interesting hits, e.g. proteins involved in human diseases, will be tested in future for hydroxylation processes.
- E) To explore potential applications of Cα hydroxylation, e.g. in Health and Enzyme Technology. Emphasis will be given, inter alia, on bacterial pathogenicity which is linked to self-hydroxylation of the conserved Pro in PDAs at the level of bacterial defense mechanisms, and on human diseases potentially linked to the hydroxylation reaction, e.g. the link between Gly Cα hydroxylation and cancer proliferation (Owen & Merkler, 2004).
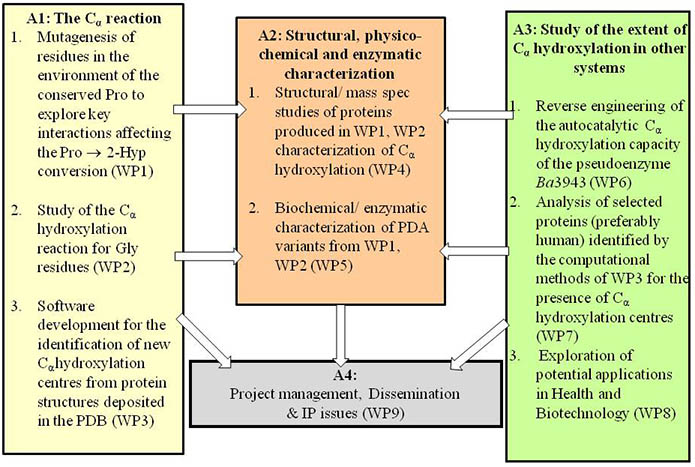
The Workpackages, Tasks and Activities of the OH-Cα project: